Where other architectural metals exhibit limited life span, titanium endures. It withstands urban pollution, marine environments, acid rain, volcanic ash residue, industrial emissions and other extremely aggressive atmospheric conditions.
Titanium and its alloys provide excellent resistance to general localized attack under most oxidizing, neutral and inhibited reducing conditions. The metal’s corrosion resistance is due to a stable, protective, strongly adherent oxide film. This film forms instantly when a fresh surface is exposed to air or moisture.The composition of this film varies from Ti02 at the surface to Ti0203 to Ti0 at the metal interface. Oxidizing conditions promote the formation of Ti02 so that in such environments the film is primarily Ti02. This film is transparent in its normal thin configuration and not detectable by visual means. This oxide film is the armor that provides the barrier protection against the environment.
A study of corrosion resistance of titanium is basically a study of the properties of the oxide film. The oxide film on titanium resists corrosion from acid rain, jet fuel residue, industrial pollution and marine environments that will typically cause pitting and corrosion of all other architectural metals. Titanium is capable of “healing” this film almost instantly in any environment where a trace of moisture or oxygen is present because of its strong affinity for oxygen.
• Sea water corrosion resistance is comparable to that of platinum – ideally suited for applications in coastal areas.
• Excellent corrosion resistance to acid rain and corrosive gases (sulfurous acid gas, hydrogen sulfide gas, etc.) – suited for applications in large cities and industrial areas.
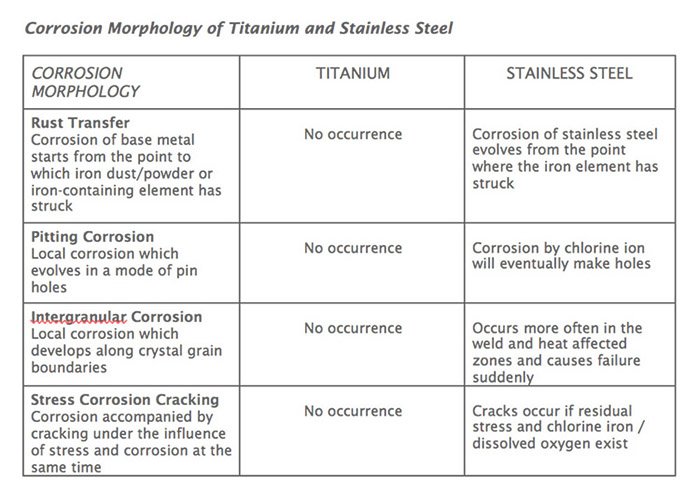
• Titanium is free of stress, pitting and crevice corrosion as well as other types of corrosion or problems inherent to stainless steel.
The term “galvanic corrosion” refers to the phenomenon that occurs when two dissimilar metals or alloys are in contact with each other in the presence of an electrolytic solution that bridges the two metals.
The coupling of titanium with dissimilar metals usually does not accelerate the corrosion of the titanium. For most architectural applications, titanium will be the cathodic member of any galvanic couple. In order to avoid problems with galvanic corrosion, it is best to isolate titanium from dissimilar metals.
GALVANIC CORROSION
The term “galvanic corrosion” refers to the phenomenon that occurs when two dissimilar metals or alloys are in contact with each other in the presence of an electrolytic solution that bridges the two metals.
The coupling of titanium with dissimilar metals usually does not accelerate the corrosion of the titanium. For most architectural applications, titanium will be the cathodic member of any galvanic couple. In order to avoid problems with galvanic corrosion, it is best to isolate titanium from dissimilar metals.


